Mouse models of two rare brain development disorders see their conditions corrected through manipulation of histone H3K4me.
2:36 PM
Author |
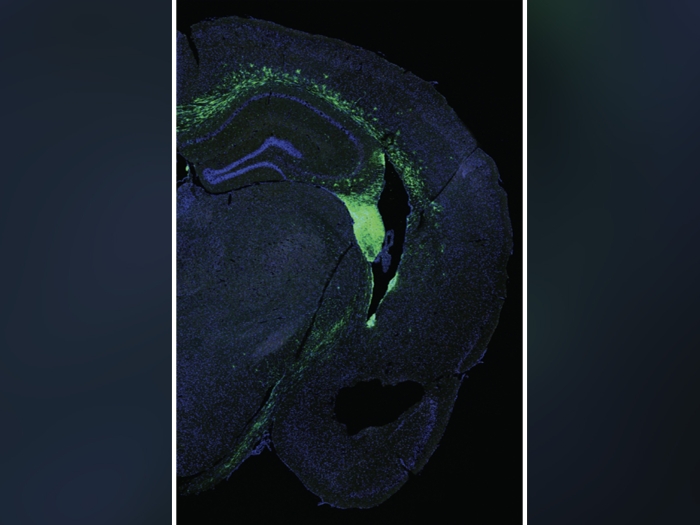
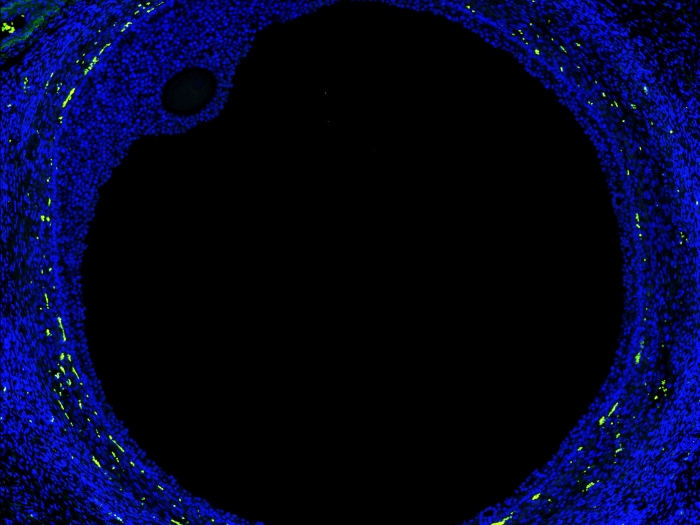
Normal brain development occurs in part through a process called histone methylation, which controls the expression of the genetic code written in DNA. One of these histones, called H3K4me, is found extensively throughout the brains of mammals, including humans. Errors in the methylation of H3K4me can cause neurodevelopmental disorders and intellectual disabilities. In a new study, Shigeki Iwase, Ph.D., Natalie Tronson, Ph.D. and their team have shown how these errors—and their corresponding memory and behavioral deficits— may be corrected in an animal.
"Mutations in many H3K4me enzymes are responsible for neurodevelopmental disorders. Yet, we do very little about how these mutations lead to brain malfunctions," says Iwase, associate professor in the U-M Medical School's department of human genetics. "Determining the functional relationship between H3K4me enzymes, therefore, is an essential first step for therapeutics of these conditions."
By manipulating so-called writer and eraser enzymes that control the methylation of H3K4me, they for the first time, reversed abnormal brain cell structure and aggressive behaviors in mouse models of two rare neurodevelopmental syndromes, Wiedemann-Steiner Syndrome and Claes-Jensen syndrome. The findings hint at the potential therapeutic promise of altering enzymes that control histone methylation in humans.
Paper cited: "Mutually suppressive roles of KMT2A and KDM5C in behaviour, neuronal structure, and histone H3K4 methylation," Communications Biology. DOI: 10.1038/s42003-020-1001-6

Explore a variety of healthcare news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!