New U-M research has shown that pluripotent stem cells can self-organize into a structure similar to the amniotic sac, an early stage of human development. The discovery could be used to study why pregnancies fail.
5:00 AM
Author |
The first few weeks after sperm meets egg still hold many mysteries.
Among them: what causes the process to fail, leading to many cases of infertility.
MORE FROM THE LAB: Subscribe to our weekly newsletter
"As many as half of all pregnancies end in the first two weeks after fertilization, often before the woman is even aware she is pregnant," says University of Michigan cell biologist Deborah Gumucio, Ph.D.
Despite the importance of this critical stage, scientists haven't had a good way to explore what can go wrong — or even what must go right — after the newly formed ball of cells implants in the wall of the human uterus.
A new achievement using human stem cells may help change that.
Tiny lab-grown structures could give researchers a chance to see what they couldn't before while avoiding ethical issues associated with studying actual embryos.
Gumucio and her U-M colleagues report in Nature Communications that they have coaxed pluripotent human stem cells to grow on a specially engineered surface into structures that resemble an early aspect of human development called the amniotic sac.
The cells spontaneously developed some of the same structural and molecular features seen in a natural amniotic sac, which is an asymmetric, hollow ball-like structure containing cells that will give rise to a part of the placenta as well as the embryo itself.
Because the structures grown at U-M lack other key components of the early embryo, they can't develop into a fetus.
It's the first time a team has grown such a structure starting with stem cells, rather than coaxing a donated embryo to grow, as a few other teams have done.
The U-M researchers hope their model will be useful to infertility researchers worldwide.
"For some couples, there is a chronic inability to get past these critical early developmental steps, but we have not previously had a model that would allow us to explore the reasons why," says Gumucio, who co-authored the report and is the Engel Collegiate Professor of Cell & Developmental Biology at Michigan Medicine, U-M's academic medical center.
"We hope this work will make it possible for many scientists to dig deeper into the pathways involved in normal and abnormal development so we can understand some of the most fascinating biology on earth."
For some couples, there is a chronic inability to get past these critical early developmental steps, but we have not previously had a model that would allow us to explore the reasons why.Deborah Gumucio, Ph.D.
A steady PASE
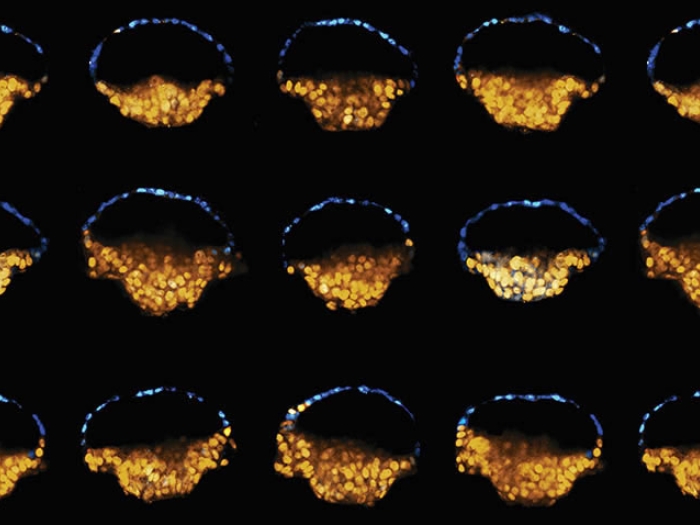
The researchers have dubbed the new structure a post-implantation amniotic sac embryoid, or PASE. They describe how a PASE develops as a hollow spherical structure with two distinct halves that remain stable even as cells divide.
SEE ALSO: A Drug Makes Stem Cells 'Embryonic' Again
One half is made of cells that will become amniotic ectoderm, the other half consists of pluripotent epiblast cells that in nature make up the embryonic disc. The hollow center resembles the amniotic cavity — which in normal development eventually gives rise to the fluid-filled sac that protects and cushions the fetus during development.
Gumucio likens a PASE to a mismatched plastic Easter egg or a blue-and-red Pokémon ball — with two clearly divided halves of two kinds of cells that maintain a stable form around a hollow center.
The team also reports details about the genes that became activated during the development of a PASE, and the signals that its cells send to one another and to neighboring tissues. They show that a stable two-halved PASE structure relies on a signaling pathway called BMP-SMAD that's known to be critical to embryo development.
Gumucio notes that the PASE structures even exhibit the earliest signs of developing a "primitive streak," although it was not fully-fledged.
In a human embryo, the streak would start a process called gastrulation. That's the division of new cells into three cell layers — endoderm, mesoderm and ectoderm — that are essential to give rise to all organs and tissues in the body.

Collaboration provides the spark
The new study follows directly from previous collaborative work between Gumucio's lab and that of the other senior author, U-M mechanical engineering associate professor Jianping Fu, Ph.D.
In the previous work, reported in Nature Materials, the team succeeded in getting balls of stem cells to implant in a special surface engineered in Fu's lab to resemble a simplified uterine wall. They showed that once the cells attached themselves to this substrate, they began to differentiate into hollow cysts composed entirely of amnion — a tough extraembryonic tissue that holds the amniotic fluid.
But further analysis of these cysts by co-first authors of the new paper Yue Shao, Ph.D., a graduate student in Fu's lab, and Ken Taniguchi, a postdoctoral fellow in Gumucio's lab, revealed that a small subset of these cysts was stably asymmetric and looked exactly like early human or monkey amniotic sacs.
The team found that such structures could also grow from induced pluripotent stem cells (iPSCs) — cells derived from human skin and grown in the lab under conditions that give them the ability to become any type of cell, similar to how embryonic stem cells behave.
This opens the door for future work using skin cells donated by couples experiencing chronic infertility, which could be grown into iPSCs and tested for their ability to form proper amniotic sacs using the methods devised by the team.
Important notes and next steps
Beyond working with genetic and infertility specialists to delve deeper into PASE biology as it relates to human infertility, the team is hoping to explore additional characteristics of amnion tissue.
SEE ALSO: Lab-Grown Human 'Mini Lungs' Successfully Engraft in Mice
For example, early rupture of the amnion tissue can endanger a fetus or be the cause of a miscarriage. The team also intends to study which aspects of human amnion formation also occur in development of mouse amnion. The mouse embryo model, Gumucio says, is very attractive as an in vivo model for human genetic disease.
The team's work is overseen by a panel that monitors all work done with pluripotent stem cells at U-M and performed in accordance with laws regarding human stem cell research. The team ends experiments before the balls of cells effectively reach 14 developmental days, the cutoff used as an international limit on embryo research — even though the work involves tissue that cannot form an embryo.
Some of the stem cell lines were derived at U-M's privately funded MStem Cell Laboratory for human embryonic stem cells and the U-M Pluripotent Stem Cell Core.
The research was funded by the National Institutes of Health and U-M. The team has worked with the U-M Office of Technology Transfer to apply for a patent on the method of generating amnion for potential commercial use in wound healing.

Explore a variety of healthcare news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!