Researchers successfully generate organoids in suspension, offering a new opportunity for investigators.
11:28 AM
Author |
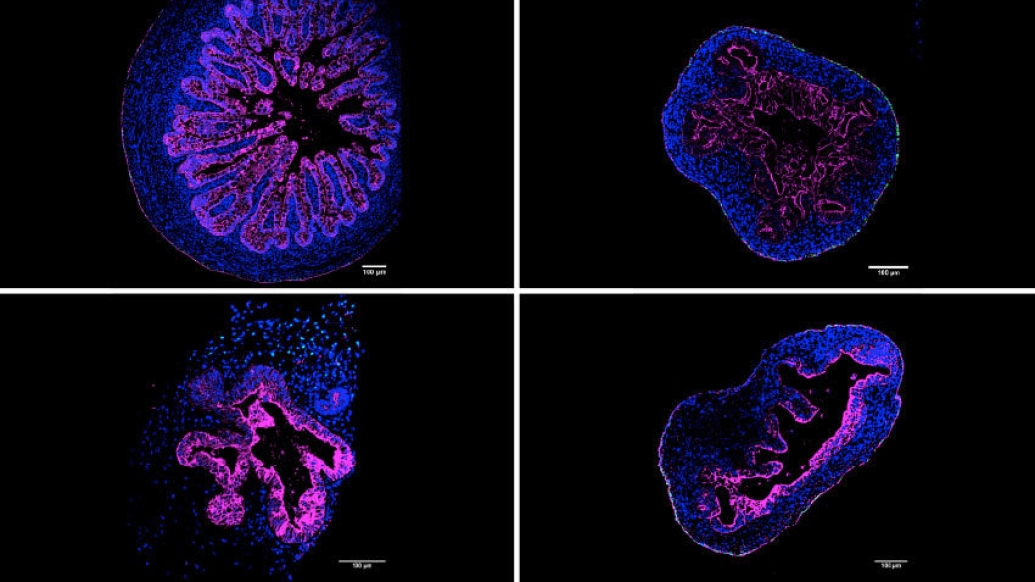
During the past ten years, scientists who study how the human body develops and functions on the most basic level have enjoyed a renaissance of sorts, thanks to structures called organoids–tiny 3D models of organs developed from pluripotent stem cells that grow in petri dishes.
Organoids are derived from human pluripotent stem cells, which can be coaxed into any cell type in the human body and have become an important research tool for understanding human development and disease. They have allowed scientists to move away from simple two-dimensional growth of cells in culture and have provided important insights into the complex three-dimensional form and function of various organs, such as the lungs, brain and heart. However, growing tiny organs in a dish is a tricky process.
A University of Michigan Medical School laboratory, headed by Jason Spence, Ph.D., of the Department of Internal Medicine and the Department of Cell & Developmental Biology, has developed a new, significantly simpler way of cultivating a 3D model of the intestine that leads to increased complexity and organization. The advance, published in Cell Reports, details how intestinal organoids now include cells that make up the serosal mesothelium, the protective, outermost layer of the intestine. This layer is also found lining many other organ systems and is critical for the production of a non-adhesive surface allowing relatively frictionless movement of organs within the abdominal cavity.
Like Podcasts? Add the Michigan Medicine News Break on iTunes, Google Podcasts or anywhere you listen to podcasts.
Past research growing various types of mini organs relied on a supportive gel called Matrigel, which forms a 3D scaffolding that allowed different cell types to develop into an organoid.
"Matrigel is the gold-standard for organoid cultures, but has limitations," explained Meghan Capeling, a Ph.D. candidate in Spence's lab and lead of the new research. For one, Matrigel is very expensive, at around $200 for 5mL of product. Second, it's derived from mouse tumor cells, "so if you're considering downstream clinical applications, it wouldn't work well because it contains unknown biological components," said Capeling.
In an earlier paper published in 2018, Capeling and her colleagues determined that intestinal organoids could be grown in a simpler, biologically inert alginate gel, as they form their own supportive mesenchymal cells—cells that in the developing fetus that turn into connective tissue and smooth muscle.
This finding led the team to wonder if the cells needed a 3D growth environment at all. The answer, they determined, is no. In the new paper, they describe their successful generation of the human intestinal organoid in a simple suspension culture.
"It actually looks somewhat like bubble tea," said Capeling, "It is just a normal tissue culture plate filled with growth media." (Growth media is a liquid with life-sustaining chemicals and nutrients for the growth of cells.) They compared the suspension organoids to actual human tissue, as well as to organoids formed using Matrigel and alginate and found that they looked similar at the molecular level. In fact, the suspension organoids more closely resembled the actual human tissue.
The team's next step was to see whether these floating mini-intestines could actually function like a developing human intestine, and they sought to use the organoids to understand how the serosal layer forms, noting that little-to-nothing is known about this process in the context of human development. The team interrogated the chemical cues that cause the serosa to form in suspension culture, says Capeling.
"This is one of the first studies to gain an idea of the specific regulators that might play a role in the proper development of the intestinal serosa."
Given that abnormal development of the serosa can lead to congenital defects, the team hoped to leverage the organoids to uncover how this tissue layer forms normally. Using drugs that block the activity of specific proteins, Capeling and the team identified two pathways, called Wnt and Hedgehog, that were essential for the normal formation of the serosa. Though the suspension method resulted in fewer organoids overall, for researchers using human pluripotent stem cells, the method could be a game-changer. The team hopes that suspension culture will open up the possibility of larger-scale organoid experiments and will be an improved system to study human development and disease.
Additional authors include Sha Huang, Charlie Childs, Joshua H. Wu, Yu-Hwai Tsai, Angeline Wu, Neil Garg, Emily M. Holloway, Nambirajan Sundaram, Carine Bouffi, Michael Helmrath and Jason R. Spence.
Paper cited: "Suspension culture promotes serosal mesothelial development in human intestinal organoids," Cell Reports. DOI: 10.1016/j.celrep.2022.110379

Explore a variety of healthcare news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!