A new discovery helps explain how multiple DNA differences can lead to development of the same disease: type 2 diabetes.
3:00 PM
Author |
Why do some people get type 2 diabetes, while others who live the same lifestyle don't?
For decades, scientists have tried to solve this mystery — and have found more than 80 tiny DNA differences that seem to either raise the diabetes risk in some people or protect others from the disease's damagingly high blood sugar levels.
MORE FROM THE LAB: Subscribe to our weekly newsletter
But no one type 2 diabetes signature has emerged from this search.
Now, a team of scientists reports a commonality among some diabetes-linked genetic defects, a discovery that might explain how multiple genetic flaws can lead to the same disease. Specifically, the flaws seem to change the way certain cells in the pancreas "read" genes.
It's the first demonstration that many type 2 diabetes-linked DNA changes have to do with the same DNA-reading molecule. Called regulatory factor X, or RFX, it's a master regulator for a number of genes.
The discovery could lead to more personalized treatments for diabetes.
The work from the University of Michigan, the National Institutes of Health, the Jackson Laboratory for Genomic Medicine, the University of North Carolina and the University of Southern California is published in the Proceedings of the National Academy of Sciences.
Many diabetes-linked DNA changes affect the ability of RFX to bind to specific locations in the genomes of pancreas cell clusters called islets, the team reports. This in turn changes the cells' ability to carry out important functions.
Islets contain the cells that make hormones, including insulin and glucagon, which keep blood sugar balanced in healthy people. In people with diabetes, that regulation goes awry — leading to a range of health problems that can develop over many years.
"We have found that many of the subtle DNA spelling differences that increase risk of type 2 diabetes appear to disrupt a common regulatory grammar in islet cells," says Stephen C.J. Parker, Ph.D., an assistant professor of computational medicine and bioinformatics, and of human genetics, at the U-M Medical School. "RFX is probably unable to read the misspelled words, and this disruption of regulatory grammar plays a significant role in the genetic risk of type 2 diabetes."
Tracking 'footprints'
To make the discovery, the researchers extensively examined DNA from islet samples isolated from 112 people. They characterized differences not only in DNA sequences, but also in the way DNA was packaged and modified by epigenetic factors and the levels of gene expression products that indicated how often the genes had been read and transcribed.
This allowed them to track the footprints that RFX and other transcription factors leave on packaged DNA after they have done their jobs.
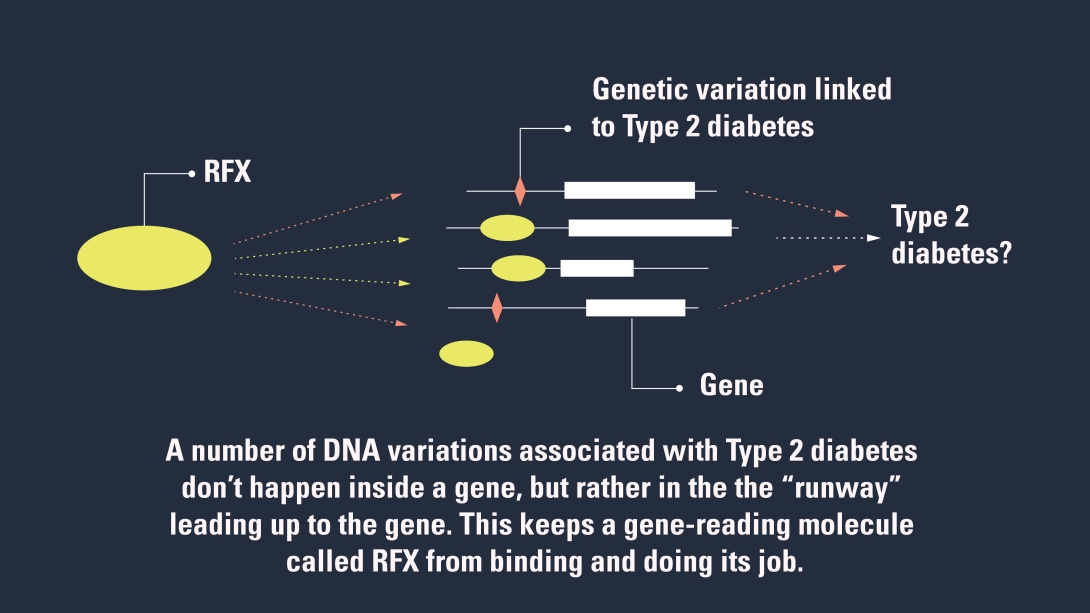
RFX and other factors don't bind directly to the part of a gene that encodes a protein that does a cellular job. Rather, they bind to a stretch of DNA near the gene — a runway of sorts.
But when genetic changes linked to type 2 diabetes are present, that runway is disrupted and RFX can't bind as it should.
Each DNA change might alter this binding in a different way, leading to a slightly different effect on type 2 diabetes risk or blood sugar regulation. The common factor for many of these changes was their effect on the area where RFX is predicted to bind, in the cells of pancreatic islets.
So, says Parker, this shows how the genome — the actual sequence of DNA — can influence the epigenome, or the factors that influence gene expression.
The researchers note that a deadly form of diabetes seen in a handful of babies born each year may be related to RFX mutations. That condition, called Mitchell-Riley syndrome, involves neonatal diabetes and a malformed pancreas and is known to be caused by a rare autosomal recessive mutation of one form of RFX.
Parker is one of four co-senior authors on the paper, which also include Michael Boehnke, Ph.D., of the U-M School of Public Health's Department of Biostatistics, Francis Collins, M.D., Ph.D., director of the National Institutes of Health, and Michael L. Stitzel, Ph.D., of the Jackson Laboratory.
Graduate student Arushi Varshney is one of the paper's co-first authors with Laura Scott, Ph.D., and Ryan Welch, Ph.D., of the U-M School of Public Health's Department of Biostatistics, and Michael Erdos, Ph.D., of the National Human Genome Research Institute.
In addition to co-senior and co-first authors listed above, the study's authors include a range of researchers from several institutions.
The study was funded by the National Institutes of Health (HL127984, DK062370, HG000024, DK099240, DK092251, DK093757, DK105561, DK072193, ZIA HG000024). Parker is a 2014 recipient of the American Diabetes Association's Pathway to Stop Diabetes grant, a type of grant awarded annually by the American Diabetes Association to provide up to $1.625 million to each scientist over a five- to seven-year grant term to spur breakthroughs in clinical science, technology, diabetes care and potential cures. Since launching in 2013 Pathway has awarded more than $36 million to 23 leading scientists.

Explore a variety of healthcare news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!