More treatment options, targeted therapies and an understanding of when less is more have improved breast cancer outcomes in recent years. But more work remains.
2:00 PM
Author |

This is a story of progress: Tremendous leaps forward that have resulted in millions of women surviving breast cancer.
MORE FROM THE LAB: Subscribe to our weekly newsletter
It's also a story of limitations. For every advance, breast cancer throws up a new barrier, creating more questions and tougher challenges.
"When are we going to cure cancer? How many times have I heard that?" says Daniel F. Hayes, M.D., Stuart B. Padnos Professor of Breast Cancer Research at the University of Michigan Rogel Cancer Center.
"Of course, one answer is we do cure many cancers. The real problem is we don't cure enough of them, and we're not curing them fast enough."
Hayes ticks off the spectrum of breast cancer: risk assessment, prevention, screening, surgery, radiation, chemotherapy, endocrine therapy, metastasis. "We've made progress in all those," he says.
Over the past 30 years, a woman's odds of dying from breast cancer have decreased by one-third. We have more than 3.3 million breast cancer survivors in the United States, according to the National Breast Cancer Foundation.
"I have patients in my clinic who have had metastatic breast cancer for 20 to 25 years. And I have many patients who we think are cured of their disease," says Max S. Wicha, M.D., Madeline and Sidney Forbes Professor of Oncology at the U-M Rogel Cancer Center.
Wicha, who founded the cancer center in 1986, cites patients with metastatic cancer who now have no signs of cancer and have not had any treatment for 10 years.
"Therapies have gotten much more specific and less toxic. Patients do better for much longer with much fewer side effects. In many cases, breast cancer has become more of a chronic disease," Wicha says.
But along with this hope and promise comes the reality that the vast majority of women with metastatic breast cancer will succumb to the disease. That's 40,610 Americans who will die of breast cancer this year, according to the American Cancer Society.
Learning that less is more
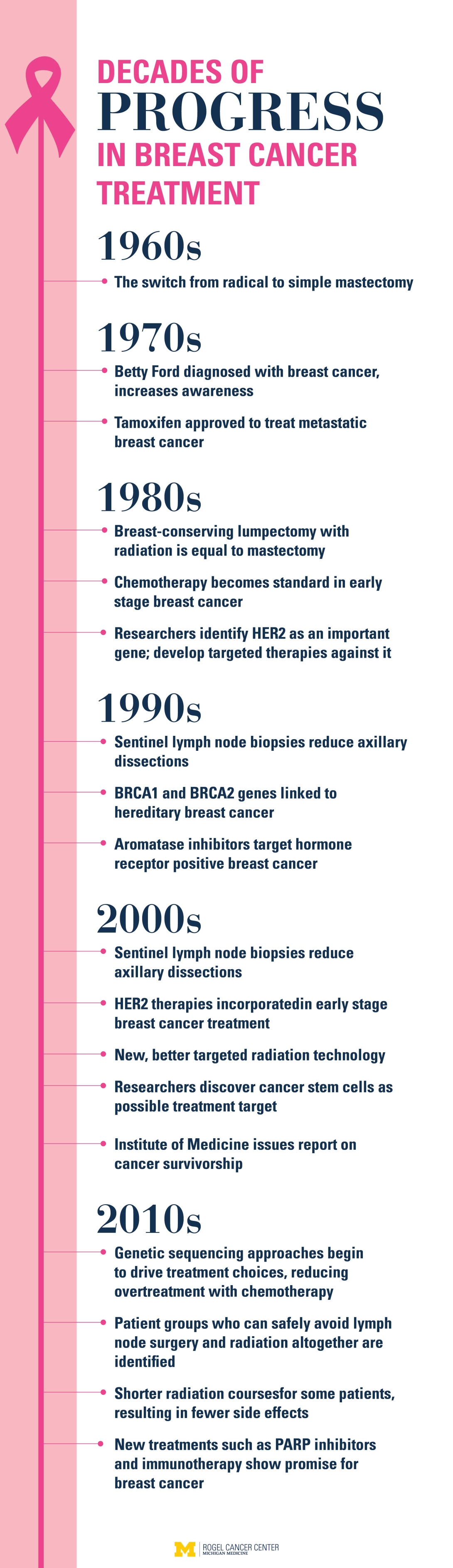
Two generations ago, when a woman was diagnosed with breast cancer, she had a radical mastectomy — a grueling procedure to remove the breast, the underlying chest muscle and the lymph nodes. This caused incredible disfigurement. A major step forward came in the 1960s when a randomized clinical trial showed that a simple mastectomy that left the chest muscle intact resulted in similar survival.
It was the beginning of realizing that sometimes less is more.
"It's hard to pull back on treatment. It takes a certain type of patient and a certain type of bravery to be willing to forgo treatment in order to help determine if that's an option for future generations," says Anne Schott, M.D., professor of internal medicine and associate director of clinical research at the U-M Rogel Cancer Center.
Over time, studies have allowed surgeons to scale back treatment even more. Lumpectomy followed by radiation is just as effective as mastectomy. Many women do not need all their axillary lymph nodes removed, a procedure that can cause severe swelling and serious infections.
"We've come to understand that radical surgical treatment is not universally beneficial," says Jacqueline Jeruss, M.D., Ph.D., director of the Breast Care Center at the U-M Rogel Cancer Center.
"We want to offer patients the best treatment possible, tailored to their disease presentation. We aim to identify a surgical plan that removes the cancer while sparing the patient the potentially avoidable and unnecessary morbidities of surgical care," she adds.
More precise radiation treatments
Similarly, radiation treatments have improved. Newer technology allows radiation oncologists to plan therapy that targets the breast but avoids the heart.
"We can outline areas at risk slice by slice on a CT scan, and then we can outline what we want to miss, like the heart," says Reshma Jagsi, M.D., D.Phil., professor and deputy chair of radiation oncology at U-M.
Add to that a surprising low-tech solution: Have the patient hold her breath. This pushes the lung up, which moves the heart farther away from the breastbone, adding another layer of protection.
An analysis of more than 1,000 breast cancer patients treated with radiation at U-M found these approaches led to excellent outcomes in terms of both preventing recurrence and reducing cardiac events.
Radiation courses are getting shorter, too. Instead of coming for daily treatments for six weeks, some patients now come for only three weeks. They get higher daily doses of radiation but less dose overall. And that has also translated into fewer side effects.
Breast cancer is many diseases
Perhaps one of the most striking discoveries is the understanding that not all breast cancers are the same. Some are fueled by estrogen or progesterone. Others are driven by a protein called HER2. And some are impacted by none of these receptors — an aggressive form called triple-negative breast cancer.
This understanding has led to targeted therapies designed to attack the mechanism that's triggering the cancer, including the endocrine-based therapies tamoxifen and aromatase inhibitors, and anti-HER2 therapies such as Herceptin.
"In 1987, we knew HER2 was associated with a worse prognosis. Now, we have such a great suite of drugs against HER2 that it's the better prognosis," Hayes says.
Precision medicine techniques allow oncologists to get even more specific when targeting treatments. At U-M, patients with metastatic cancer can have their DNA sequenced, along with tumor RNA, to uncover the molecular drivers of their particular tumor. This information might suggest one drug over another.
Some patients with early stage disease can avoid chemotherapy altogether. A test called Oncotype Dx can indicate if the tumor is aggressive or slower-growing. The results help clarify who's really likely to benefit from chemotherapy and who can skip it.
"We've gone from a place where we know chemotherapy treatment helps people, to understanding that treatment helps certain people," Schott says.
Now the key is to understand which treatments will help which people the most. Who can avoid treatment? And who needs more aggressive treatment to prevent recurrence or metastasis?
Challenges still loom
This question will be the next challenge in breast cancer research. It's not well understood why some women treated for early stage breast cancer are cured while others soon find cancer has spread throughout their body. And still others think they have made it out of the woods to find cancer return 10 or even 20 years later.
"The largest vexing problem in my clinic is tumor dormancy and late relapses," Schott says.
Initially, endocrine therapy was recommended for five years. Then studies found that the drugs continued to be effective at preventing recurrence when taken for 10 years. But when patients stop their endocrine therapy after 10 years, some — but not all — will relapse.
"We don't know who has residual cancer. So the choices are stop the drug and hope it goes OK, or continue taking the drug indefinitely," Schott says.
Endocrine therapy can cause side effects such as hot flashes or joint pain. Because of this, up to half of patients choose not to stay on it, and as many as a third never start it. For some, the side effects simply outweigh the potential benefit. The goal, Schott says, is to understand who stands to gain the most benefit.
Researchers are examining circulating tumor cells — cells that detach from the tumor and travel through the bloodstream — to try to discover differences in tumor biology from one patient to the next. Through a blood test, oncologists can tally the number of circulating tumor cells and begin to predict outcomes. They hope these cells will yield clues that might predict which patients need continued therapy.

Partnering with engineers to solve metastasis
Another major challenge is treating metastatic disease, or breast cancer that has spread beyond the breast and lymph nodes. The average survival for these patients is about two years. But this average includes patients who live for many years with metastatic disease managed with treatments.
A tiny fraction of patients may be cured of metastatic breast cancer after completing treatment, with no evidence of cancer for the remainder of their normal lives. But these are rare exceptions.
What puzzles scientists is why. Those who responded had the same treatment as those who did not respond. So what makes cancer incurable?
Researchers are looking for new perspectives to better understand why cancer spreads, to try to stop the process or detect it early.
Surprisingly, engineering is playing a big role in research to understand and treat metastasis. Jeruss and Lonnie Shea, Ph.D., professor and William and Valerie Hall chair of biomedical engineering at U-M, are working on a small implantable scaffold device designed to capture cancer cells as they begin to travel through the body.
The scaffold, made of FDA-approved material commonly used in sutures and wound dressings, is designed to mimic the environment in other organs before cancer cells migrate there. It attracts the body's immune cells, and the immune cells draw in the cancer cells. By trapping the immune cells, the scaffold limits them from heading to the lung, liver or brain, where breast cancer commonly spreads.
"We need to change what we perceive is possible when we think about early detection of metastasis," says Jeruss, an associate professor of surgery, pathology and biomedical engineering. "We can use engineering concepts to detect metastases at the earliest time point and then deliver drugs or targeted treatment to the patient before the metastases have established themselves within organs."
Engineers are also building microfluidic devices that allow researchers to assess individual cells from a tumor. In a new study, researchers showed they could isolate the leader cells — those cells that first break away and migrate to other parts of the body.
They hope to find differences in the molecular signature between cells that invade and those that don't. Then, they would target the molecular underpinning with therapies to prevent cancer from invading — essentially keeping the cancer confined and preventing metastasis.
Microfluidic devices are also helping researchers assess individual cells within a tumor to identify and assess treatment options, monitor genetic changes in tumors and flag the presence of aggressive cancer stem cells.
Targeting cancer stem cells
Researchers at the U-M Rogel Cancer Center discovered in 2003 that a small number of cells within a tumor are responsible for fueling its growth and spread. These cancer stem cells do not respond to traditional chemotherapies, which kill the bulk tumor cells only.
Clinical trials at U-M are testing drugs designed to target the cancer stem cells, using them in combination with other treatments to try to improve outcomes.
"Women with advanced disease treated with Herceptin or other anti-HER2 therapies do well for a while, but eventually, they all become resistant to the HER2 blockade. Why is that? Our laboratory showed the stem cells activate the inflammatory pathway IL6. That drives the stem cells and allows them to overrule Herceptin," Wicha says.
This led to a clinical trial using tocilizumab, a drug approved for arthritis, which works by blocking IL6. In another trial, patients are randomized to receive chemotherapy or chemotherapy plus reparixin, a drug designed to target cancer stem cells through another inflammatory pathway.
Research suggests that cancer stem cells may be especially potent in triple-negative breast cancer, which represents about 15 percent of diagnoses. Because this subtype does not express receptors to estrogen, progesterone or HER2, the targeted therapies that work so well in those types are not effective in triple-negative tumors.
"New therapeutic targets and treatment strategies are crucial to improve outcomes for women with this aggressive breast cancer subtype," Jeruss says. Her research has examined a drug called CYC065, which was seen in laboratory research to show promise for the treatment of triple-negative disease.
I want to be able to say to a woman with metastatic breast cancer, we have the treatments that will cure you. That's my hope for the future.Anne Schott, M.D.
Limited success with immunotherapy
Breast cancer is in many ways ahead of other solid tumors in terms of targeted therapies. But one of the most exciting recent advances in cancer research has largely passed by breast cancer.
Immunotherapy has had tremendous success in melanoma, kidney cancer, lung cancer and prostate cancer. But no immunotherapy drugs have been approved for breast cancer. Unlike melanoma, breast cancer does not seem to generate much of an immune response.
Wicha and others at U-M are taking a number of approaches to make breast cancer cells more immune responsive. Research has found the immune system plays a role in cancer stem cells. The thinking is to create a vaccine against the cancer stem cells, rather than against all the cancer cells.
Improving quality of life for survivors
Ultimately, the goal is to help more women survive breast cancer (if not avoid it altogether). But as more women survive, more attention needs to be paid to the long-term impact of treatment.
Neuropathy, lymphedema, pain and sexual dysfunction can impact survivors for years after treatment ends. About a third of women experience moderate to severe fatigue up to 10 years later.
"One of the things I like most about treating breast cancer is so many of my patients do well long term," Jagsi says. "But what that means is we can't just look five years into the future with these patients. We have to be mindful of the toxicities and the burden we're causing to our patients."
Oncologists are beginning to monitor for many of these side effects early on so they can be addressed before becoming major issues.
Breast cancer can also have a major impact on a person's employment and financial situation — not just during treatment, but for years after. One study found that 30 percent of women who were working when they were diagnosed were unemployed four years later.
"I just don't think anyone expected that," Jagsi says. "We're doing so much better in so many ways at treating breast cancer, but a substantial number of patients are seeing true financial devastation from our treatment. We have an obligation to develop interventions that are inexpensive and scalable."
Decision aids to help women understand when they do and do not need treatment could help. And a better understanding of how to tailor treatment to those at greatest risk will allow those at lower risk to avoid the physical and financial impact of treatment.
This goes hand in hand with many recent advances. How do we intensify treatment for those who need it and limit treatment (and its burden) for those who don't?
Hopes for the future
So what lies ahead in the crystal ball? Researchers' hopes include better targeted therapies, better tests to detect breast cancer, better ways to identify aggressive tumors and deliver treatment to the right patients, more funding for research and more women willing to participate in clinical trials.
"I want to be able to say to a woman with metastatic breast cancer, we have the treatments that will cure you. That's my hope for the future," Schott says.
Learn more about breast cancer and breast cancer treatment at the U-M Rogel Cancer Center

Explore a variety of healthcare news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!